In July InnovaTalk Webinar hosted by the VinFuture Foundation, Professor Pascale Cossart (Pasteur Institute, Perpetual Secretary of the Academy of Sciences France, and Member of the VinFuture Prize Council), Dr. Nassos Typas (Group Leader and Senior Scientist at European Molecular Biology Laboratory Heidelberg Germany), Dr. Chung The Hao (Wellcome International Training Fellow and Postdoc Scientist at Oxford University Clinical Research Unit Ho Chi Minh), and Dr. Tran Thi Thanh Tam (Senior Lecturer at University Of Science And Technology Hanoi) highlighted the most recent research endeavors in understanding the interaction between medication drugs and the human gut microbiome, their implications on various complications including Antimicrobial Resistance and malnutrition, and challenges and opportunities to expand microbiome research in Vietnam.
Understanding medication-gut microbiome interactions
In his presentation, Dr. Nassos Typas began by sharing interesting statistics and notable progress in microbiome research. The human microbiome contains various elements, including bacteria, viruses, and fungi. According to various estimations, the number of cells in the human microbiome system, including in the gut, skin, and airway, exceeded the number of human cells. The number of genes in the human microbiome is over a hundred times that of the human genome, implying a wide range of functions that these commensal livings can support our body. With recent single-cell and high-throughput sequencing technology, we can understand the genomic composition of the microbiome and associate the changes in this composition with various diseases.
However, as the current research is heavily data-driven, there is a lack of mechanistic research to understand the causal relationship between the human microbiome and our well-being. Recognizing this gap in knowledge, Dr. Typas and his group established an in vitro system (an experimental approach performed in a test tube, culture dish, or elsewhere outside a living organism), in which over 100 most common species of microbes in the human gut were isolated and cultured in a large scale, allowing researchers to perform mechanistic research on these bacteria. With this system, the Typas group is further investigating three main problems.
Collateral damage of antibiotics on the human gut microbiome: antibiotics are widely used to treat bacterial infectious diseases. Even though it is intended to target foreign pathogens in certain organs, most oral drugs end up in the gut, causing its effect on the microbial system here. Perhaps unsurprisingly, 78% of FDA-approved antibiotics exhibited some level of inhibition to gut bacteria. However, their effects are highly diverse. Quinolone, a broad-spectrum antibiotic, showed a broad range of effects across samples. Meanwhile, macrolide and tetracycline showed highly consistent behavior in limiting bacterial growth.
This observation raised a question of how we can overcome the collateral damage caused by antibiotics on the gut microbiome. To answer this question, one innovative solution proposed by the Typas group is to use a selective antidote along with antibiotics to limit antibiotics’ impact on commensal microbes while maintaining deleterious effects on pathogens. In his group’s Nature paper in 2021 (Maier, Goermans, et al.), they screened for the molecule Dicumarol, an experimental anti-coagulant, to be used in conjunction with antibiotics Doxycycline and Erythromycin to selectively target pathogenic species. This served as a proof-of-concept for the innovative solution of using selective antidotes to protect the human gut microbiome from collateral damage.
Impact of non-antibiotic drugs on the human gut microbiome: Besides the direct effect of antibiotics on bacteria, non-antibiotic drugs also caused significant changes in the composition of the gut microbiome. According to observations made by Dr. Maier, a postdoctoral researcher in Typas Lab, 24% of non-antibiotic drugs, all approved by the FDA, exhibited some degree of inhibition to gut bacterial species. Even though some drugs exhibited broad effects similar to antibiotics, most showed highly specific behavior in inhibiting or eradicating bacteria. Based on these staggering observations in vitro, in vivo research is needed to confirm the similar effect in living organisms and elucidate the mechanism of certain drugs’ side effects through their impact on the gut microbiome.
Emergent communal behavior in response to medication: One of the key differences between the in vitro system employed by the Typas group compared to the actual environment in our body is that the microbes behave in a communal fashion in our body. To understand the communal effects of gut microbes, Typas Lab co-cultured 32 different common species of bacteria and tested for their response to 30 different medications. They observed the broad protection behavior across most species, highlighting the communal protective effect. However, as they increased the drug concentration, the communal protection started to collapse, implying a threshold level in enduring stress by the microbial system.
Antimicrobial resistance and challenges to microbiome research in Vietnam
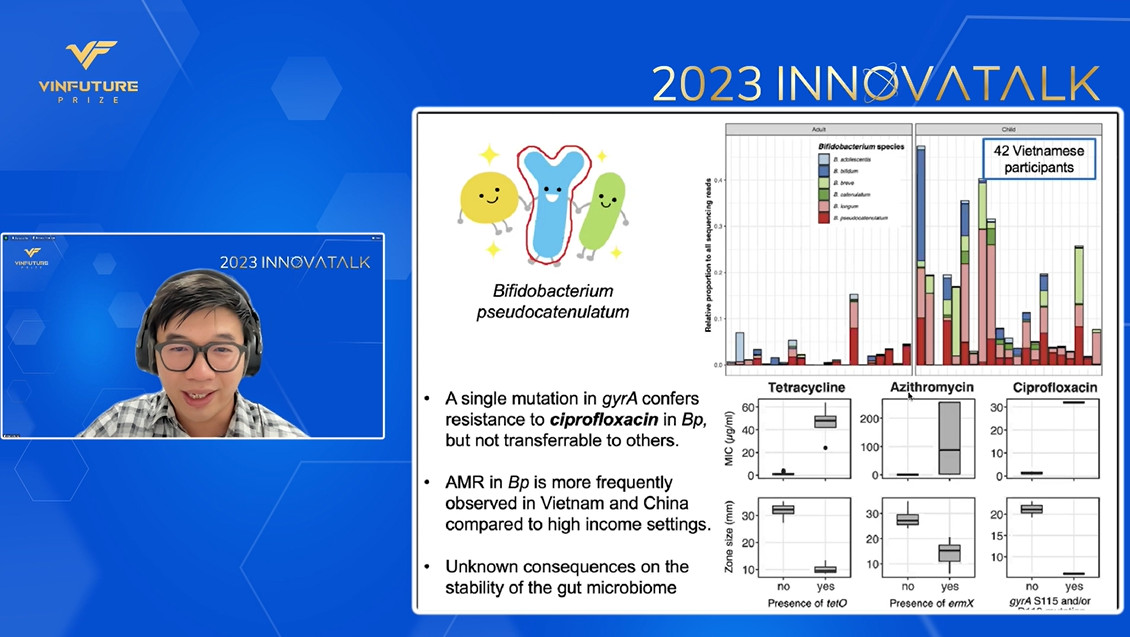
Built upon Dr. Typas’ presentation, Dr. Chung The Hao delved into a specific problem of Antimicrobial Resistance (AMR) in the context of Vietnam, the role of the gut microbiome in this complication, and challenges to microbiome research in Vietnam. Dr. Hao highlighted an important biological phenomenon among bacteria called conjugation. During that process, they can transfer the plasmid containing genes for AMR to other bacteria within the same bacterial hierarchy or having a close evolutionary relationship. This phenomenon is shown in Duy Pham et al. paper on Nature Microbiology, reporting a similar signature between AMR plasmids of commensal E. coli and S. sonnei in the gut of an infected child.
A worrisome observation from this work was that rate of AMR gene transfer among gut microbes increased as the dose of antibiotic ciprofloxacin increased, implying the worsened resistance due to the overuse of antibiotics and cooperation in the human gut microbiome. Beyond the report of a single case, Dr. Hao’s current work in Bifidobacterium Pseudocatenulatum, a common bacterium in Vietnamese adult’s gut system, showed the high prevalence of mutation in gyrA gene conferring AMR and increased rate of AMR observed in this bacterium in Vietnam and China compared to that of high-income countries.
Overall, the problem of AMR in Vietnam is deeply concerning and progressing negatively. To help slow down this trend and understand more about the impact of the human microbiome on this complication, Dr. Hao pointed out the need for investments in funding, infrastructure, expertise, and translational resources in microbiome research to produce meaningful interventions to this looming public health crisis.
Importance and uniqueness of microbiome research in Vietnam
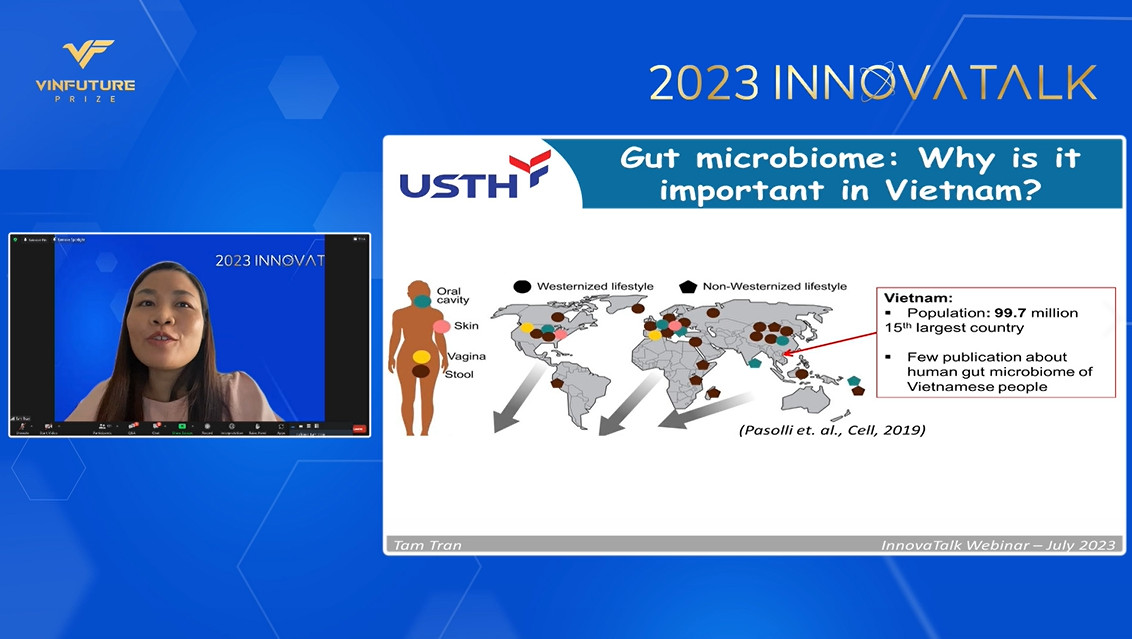
To conclude the webinar, Dr. Tran Thi Thanh Tam shared insightful perspectives on the importance and uniqueness of microbiome research in Vietnam. She highlighted the lack of large-scale microbiome characterization efforts in Vietnam and other low- and middle-income countries. Such research is essential due to Vietnamese individuals’ distinct gut microbiome profile, which is influenced by their unique diets, lifestyles, and environmental exposures compared to regions like the United States, the European Union, and China.
To realize these research efforts, Dr. Tam stressed the need for stronger funding, infrastructure, and human resources investments. Additionally, she emphasized the importance of new guidelines for microbiome research tailored to the Vietnamese context to enable better data collection and result interpretation.
Overall, the July InnovaTalk Webinar has provided valuable insights into the intricate relationship between medication drugs and the human gut microbiome. By understanding these interactions and the potential implications on health, researchers can pave the way for innovative interventions and informed healthcare practices. The collaborative efforts of experts in the field and the active participation of scholars and specialists demonstrate the growing commitment to advancing microbiome research in Vietnam and the world.